Unraveling the Composition of Talc: A Comprehensive Exploration
Related Articles: Unraveling the Composition of Talc: A Comprehensive Exploration
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Unraveling the Composition of Talc: A Comprehensive Exploration. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Unraveling the Composition of Talc: A Comprehensive Exploration
- 2 Introduction
- 3 Unraveling the Composition of Talc: A Comprehensive Exploration
- 3.1 The Chemical Composition of Talc: A Closer Look
- 3.2 The Crystal Structure of Talc: A Key to Its Properties
- 3.3 Variations in Talc Composition: Implications for Applications
- 3.4 The Importance of Purity in Talc
- 3.5 Applications of Talc: A Diverse Spectrum
- 3.6 Addressing Concerns Regarding Talc: A Focus on Safety
- 3.7 FAQs About Talc: Addressing Common Questions
- 3.8 Tips for Using Talc Safely and Effectively
- 3.9 Conclusion: The Enduring Importance of Talc
- 4 Closure
Unraveling the Composition of Talc: A Comprehensive Exploration
Talc, a soft, white mineral, has been a staple in various industries for centuries. Its unique properties, including its softness, lubricity, and inertness, have made it an indispensable component in diverse applications, ranging from cosmetics and pharmaceuticals to industrial manufacturing. Understanding the composition of talc is crucial for appreciating its versatility and potential applications.
The Chemical Composition of Talc: A Closer Look
Talc’s chemical formula, Mg3Si4O10(OH)2, reveals its primary components: magnesium, silicon, oxygen, and hydrogen. It is essentially a hydrated magnesium silicate, with the following breakdown:
- Magnesium (Mg): Magnesium is a light, silvery-white metal that plays a vital role in talc’s structure and properties. It contributes to the mineral’s softness and lubricity.
- Silicon (Si): Silicon, a metalloid, forms the backbone of the silicate structure, providing stability and strength.
- Oxygen (O): Oxygen is a highly reactive element, forming the basis of the silicate structure and contributing to talc’s overall chemical composition.
- Hydrogen (H): Hydrogen atoms are bound to oxygen atoms, forming hydroxyl groups (OH) that play a crucial role in talc’s hydration and softness.
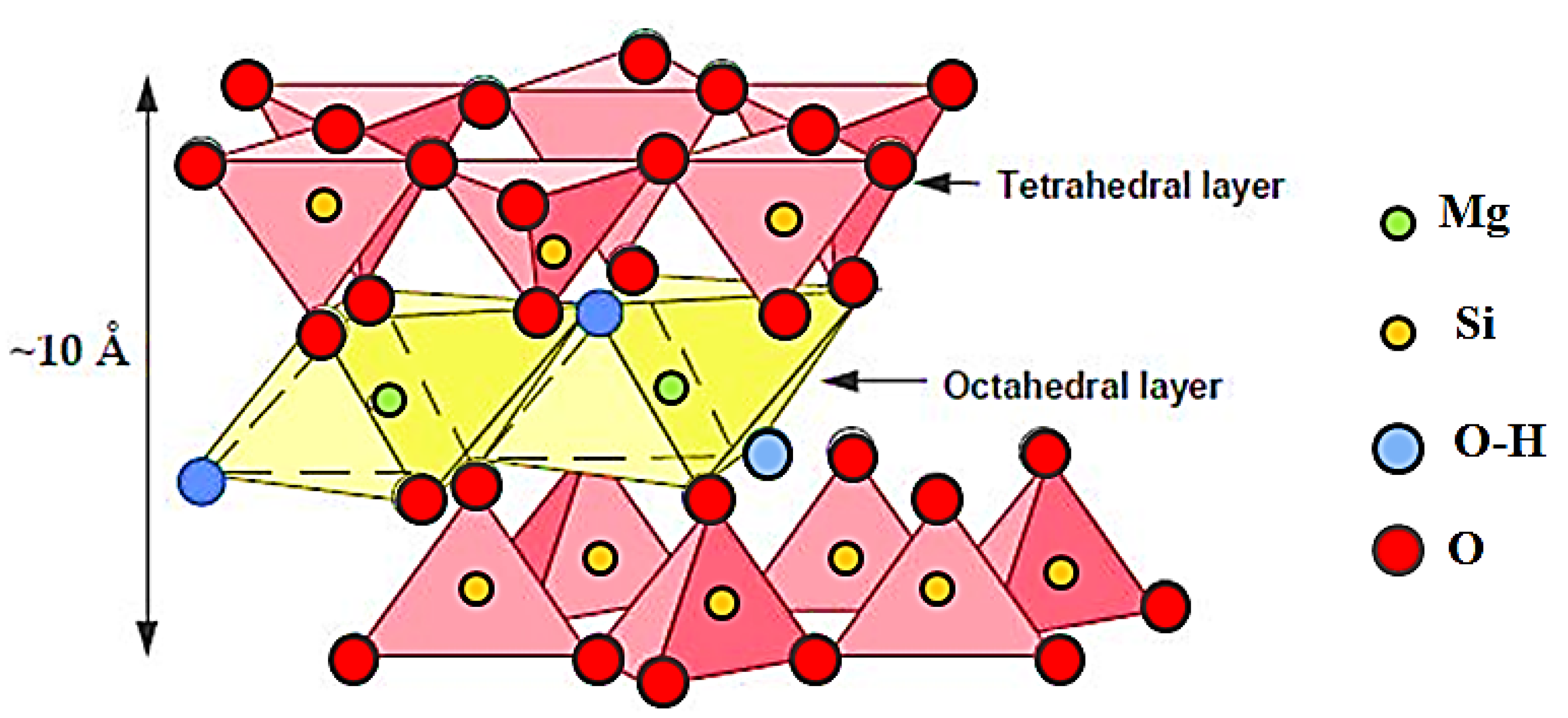
The Crystal Structure of Talc: A Key to Its Properties
Talc’s unique properties stem from its distinctive layered crystal structure. The basic unit of the structure is a sheet of silica tetrahedra, where each silicon atom is bonded to four oxygen atoms. These silica sheets are stacked on top of each other, with magnesium ions located between them. The layers are held together by weak van der Waals forces, which allow them to easily slide past each other. This layered structure explains talc’s softness and lubricity.
Variations in Talc Composition: Implications for Applications
While the basic chemical formula of talc remains constant, variations in trace elements and impurities can occur, influencing its properties and applications. These variations can be attributed to the geological conditions during talc formation. For instance, the presence of iron can impart a greenish hue to talc, while the presence of manganese can lead to a pink coloration.
The Importance of Purity in Talc
The purity of talc is a critical factor in determining its suitability for different applications. High-purity talc, with minimal impurities, is preferred for applications where purity is paramount, such as in cosmetics and pharmaceuticals.
Applications of Talc: A Diverse Spectrum
Talc’s unique properties have made it a valuable resource in various industries, including:
- Cosmetics: Talc’s softness and absorbency make it an ideal ingredient in powders, foundations, and eyeshadows. It helps absorb excess oil, provide a smooth finish, and enhance the texture of cosmetic products.
- Pharmaceuticals: Talc’s inertness and lubricity make it a valuable excipient in tablets and capsules, facilitating smooth processing and preventing sticking during manufacturing.
- Industrial: Talc’s lubricity is utilized in various industrial applications, including as a lubricant in metalworking, as a filler in paints and plastics, and as a component in ceramic glazes.
- Paper: Talc is added to paper to enhance its smoothness, opacity, and printability.
- Food: Talc can be used as a release agent in food processing, preventing sticking and facilitating smooth product flow.
Addressing Concerns Regarding Talc: A Focus on Safety
While talc is generally considered safe for most applications, concerns have been raised regarding its potential health risks. These concerns primarily center around the presence of asbestos, a known carcinogen, in some talc deposits.
It is crucial to note that not all talc contains asbestos. Talc used in cosmetics and pharmaceuticals undergoes rigorous testing to ensure it is asbestos-free. However, it is essential to choose products from reputable manufacturers who prioritize safety and quality control.
FAQs About Talc: Addressing Common Questions
1. Is talc safe to use in cosmetics?
Talc used in cosmetics is rigorously tested to ensure it is asbestos-free. However, it is always advisable to choose products from reputable manufacturers who adhere to strict quality control standards.
2. Can talc cause cancer?
The potential health risks associated with talc are primarily linked to the presence of asbestos. Pure talc, free from asbestos, is generally considered safe.
3. What are the benefits of using talc?
Talc offers various benefits, including its softness, absorbency, lubricity, and inertness. These properties make it suitable for a wide range of applications, including cosmetics, pharmaceuticals, and industrial manufacturing.
4. How is talc mined and processed?
Talc is mined from open-pit or underground mines. After extraction, it undergoes processing to remove impurities and achieve the desired particle size and purity.
5. What are the alternatives to talc?
Alternatives to talc include cornstarch, rice powder, and kaolin clay. However, each alternative has its own unique properties and limitations.
Tips for Using Talc Safely and Effectively
- Choose products from reputable manufacturers: Ensure the products you choose have undergone rigorous testing to ensure they are asbestos-free.
- Read product labels carefully: Look for products that explicitly state they are asbestos-free.
- Avoid using talc in areas prone to moisture: Talc can clump when exposed to moisture, potentially leading to irritation.
- Store talc in a cool, dry place: Proper storage helps maintain the quality and effectiveness of talc.
- Consult with a healthcare professional: If you have any concerns about using talc, consult with a doctor or dermatologist.
Conclusion: The Enduring Importance of Talc
Despite the concerns surrounding its potential health risks, talc remains an important mineral with a wide range of applications. Its unique properties, including its softness, lubricity, and inertness, make it a valuable ingredient in cosmetics, pharmaceuticals, and various industrial processes. By understanding the composition and properties of talc, and by choosing products from reputable manufacturers, consumers can utilize this versatile mineral safely and effectively. As research and technology continue to advance, we can expect to see further innovations in the use of talc, ensuring its continued relevance in various industries.


![Simplified illustrated structure of talc showing the [MgO6] octahedral](https://www.researchgate.net/publication/346330699/figure/download/fig5/AS:963547735547911@1606739090172/Simplified-illustrated-structure-of-talc-showing-the-MgO6-octahedral-sheet-sandwiched.png)


Closure
Thus, we hope this article has provided valuable insights into Unraveling the Composition of Talc: A Comprehensive Exploration. We hope you find this article informative and beneficial. See you in our next article!